Graphite ore is one of the crystalline minerals of carbon. It has excellent properties such as lubricity, stability, high-temperature resistance and electrical conductivity. With the rise of new energy and new materials industries, graphite has become more and more widely used as an important non-metal.
The properties and uses of graphite ore depend on its crystal morphology. There are roughly three types of natural graphite ores: block graphite ore, flake graphite ore and aphanitic graphite ore. The crystalline morphology and grade of these three types of graphite ores are different, and the graphite ore beneficiation processes are also different. Block graphite ore has high grade, generally up to 60%~65%, or even up to 80%~98%, which is little need for mineral processing. The flake graphite ore and aphanitic graphite have the relatively low relative grade and need to be processed by graphite ore beneficiation process.

Use the table of contents below to navigate through the guide:
01Composition, Properties and Distribution of Graphite Ore
The main component of graphite ore is carbon. Natural graphite ore usually has 10% to 20% impurities, and there is few pure graphite ores. The impurities include silicon dioxide, alumina, magnesium oxide, calcium oxide, phosphorus pentoxide, copper oxide, vanadium oxide, hydrogen oxide, sulfur, ferrous oxide, hydrogen, nitrogen, carbon dioxide, methane, ammonia, etc. Graphite ores are usually iron-black and steel-gray with metallic luster, while aphanitic graphite ore is dim and opaque.
Graphite ore is chemically stable, which is free from acid, alkali, and organic solvents. It has high-temperature resistance, electrical conductivity, thermal conductivity, and good lubricity and plasticity.
Graphite reserves in Brazil, China, India and Mexico account for 92.77% of the world's total reserves. China's basic graphite reserves account for about 33% of the world's total, second only to Brazil (about 38% of the world). Brazil's graphite ore reserves are 58 million tons, India 11 million tons of graphite and Mexico 3.1 million tons of graphite.

02Types of Graphite Deposits
(1) Graphite ore is disseminated and scaled in volcanic rocks and siliceous sedimentary rocks. Such deposits have large graphite scales and high ore quality, and there are famous Madagascar scaly crystalline graphite deposits.
(2) Graphite-bearing ores are vein-filled in fractures and caves. The grade of graphite in these deposits is high, and the typical deposit is vein-like graphite in Sri Lanka.
(3) Hydrothermal metasomatic contact metamorphic deposits are formed by the intrusion of intermediate-acid and acid granites into marble. Such ore deposits have good ore quality and are distributed in countries such as Russia, North Korea and other countries.
(4) The graphite in the ore of metamorphic graphite deposits in coal or carbon-rich sediments is mostly cryptocrystalline. Most of the graphite deposits in Mexico, India and Australia belong to this type.

03Flake Graphite Ore Beneficiation Process
Flake graphite ore has a special structure, the larger the scale, the higher the carbon content, the greater the use-value. Therefore, in the process of graphite grinding separation, it is necessary to protect the large scale of graphite from being destroyed.
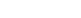
Different grinding medium has different modes of contacting minerals, so their grinding efficiency has different protection effects on graphite ore. The steel ball, steel rod and steel column have their own advantages, among which the steel rod plays an important role in protecting the large-scale graphite ore, but the grinding efficiency is not high. When the steel rod works on the grinding mill, the steel rod contacts the mineral as surface-to-surface contact, so the coarse mineral can be grounded firstly, and the large-scale graphite ore is protected. In the actual production, increasing the mill speed appropriately can solve the problem of low grinding efficiency. In addition, using the vertical agitating mill can effectively protect the large scale of graphite ore, and improve the concentrate grade.


 marketing@ytxinhai.com
marketing@ytxinhai.com  0086 13810327080
0086 13810327080 
































































































 CHAT
CHAT MESSAGE
MESSAGE